

10 best countries to outsource software development
Software engineering outsourcing is an efficient budget optimization strategy that allows picking the most competent contractor. You can hire a qualified team suitable for both a startup and a global marketplace product. Check out the list of the best outsourcing countries 2022 and the specifics of organizing a working project with a team from abroad.
Outsource software development: What does it mean?
Outsourcing is a business opportunity to transfer certain project functions to a third-party contractor. In other words, the company temporarily orders particular services or support for some tasks to focus on the main project objectives, saving time, and financial resources. Outsourcing is relevant for small-, medium-sized, and large businesses alike.
Are you looking for a reliable software vendor? Check what we do.
Reasons why companies outsource software development
The list of niches where outsourcing collaboration with a team is effective is wide: the energy sector, healthcare, pharmaceuticals, tourism, retail, and so on. Outsourcing of IT services is a great solution for digital transformation or automation of business processes. Below are six reasons indicating the advantage of working with an outsourced team.
Reason 1. Minimized workplace organization (and recruitment) expenses
According to the Deloitte study, about 59% of companies choose outsourcing to reduce or optimize project costs. How exactly are they looking to save? The thing is in-house developers require a comfortable working environment with high-performance equipment and convenient furniture. Every hired employee thus spawns expenses for setting up a workplace. IT outsourcing eliminates this issue.
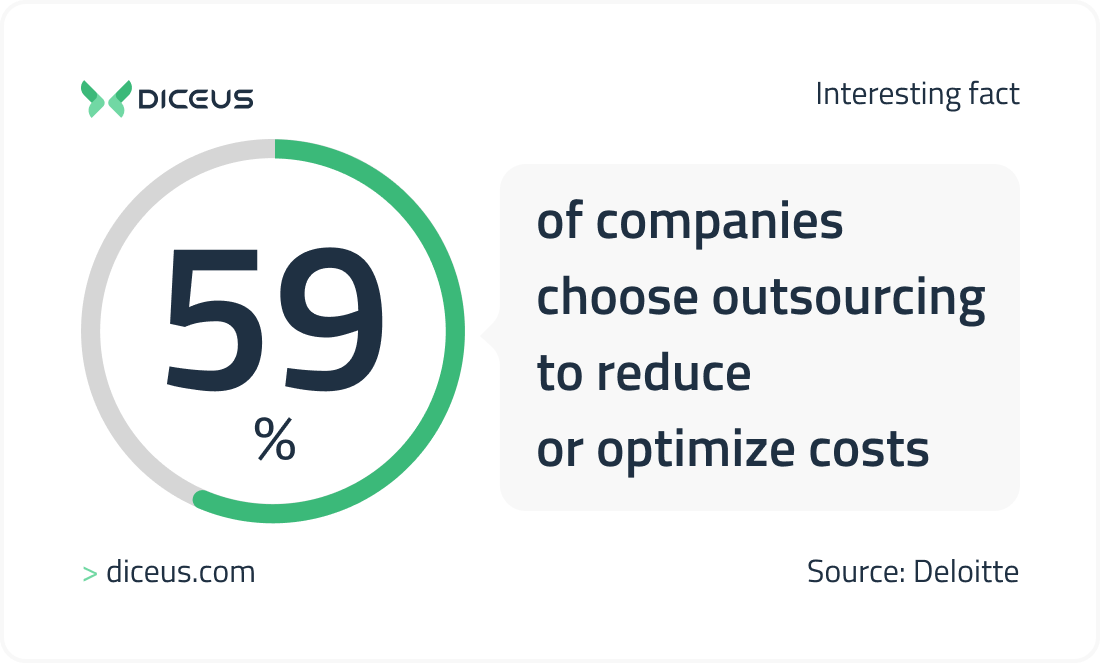
You pay strictly for the time developers work on the project. At the same time, when choosing offshore development, the hourly rate of specialists is mostly lower than in your country. There is also no need for any costs associated with recruiting and motivating the team, creating a corporate culture, and building employer branding.
Reason 2. An ability to hire a full team or separate specialists
Outsourcing allows picking between hiring a complete team or specific specialists. If your company has thousands of employees, you can probably afford to hire a software development team. If you are a young, small startup, and you are looking for investors or have not yet launched a marketplace product, hiring several developers is always an option when it comes to outsourcing.
500 Startups founder Dave McClure said that the perfect startup team is one that consists of three types of people:
- Those who can code (hackers)
- Those who understand industry specifics and can manage a business properly (hustlers)
- And designers that build a project carcass
Reason 3. Involvement of qualified experts
The ultimate success of a product depends a lot on hiring the right people. You need to be careful who you hire from the very beginning. Candidates must have a background in your industry, technical knowledge, ad-hoc problem solving, good communication skills, and a lively interest in your product. The last criterion is important for new employees to understand, develop, and present the project mission.
Reason 4. Adaptation time is short
Most companies providing IT outsourcing services have teams of developers, designers, product and project managers, and other specialists who have jointly closed at least several projects.
When attracting new people to the in-house staff, there is a risk of product development delays, since new employees need time to adapt to new working conditions and get used to other team members. In turn, this problem doesn’t arise when hiring an outsourced team.
Reason 5. Fewer risks
Yet another advantage of working with a team from one of the top outsourced countries is lower overall risks. For instance, the qualifications of a staff team are often limited to a range of routine tasks. Cooperation with freelancers is often an unreliable solution because they can fail to meet the deadlines and lack real knowledge in the area targeted by the client.
By outsourcing IT services, you automatically reduce these potential risks. The selection of a qualified team and optimization of processes allow eliminating many issues at an early stage of development in compliance with all deadlines.
Reason 6. Focus on the main business tasks
Outsourced dev teams use a variety of project management and tracking approaches such as Waterfall or Scrum. The client can clarify the details or the stage of implementation with the person responsible, but direct participation in the project is minimal. By freeing up time, you can focus on core business tasks rather than manage a software development team yourself.
Summarizing the reasons
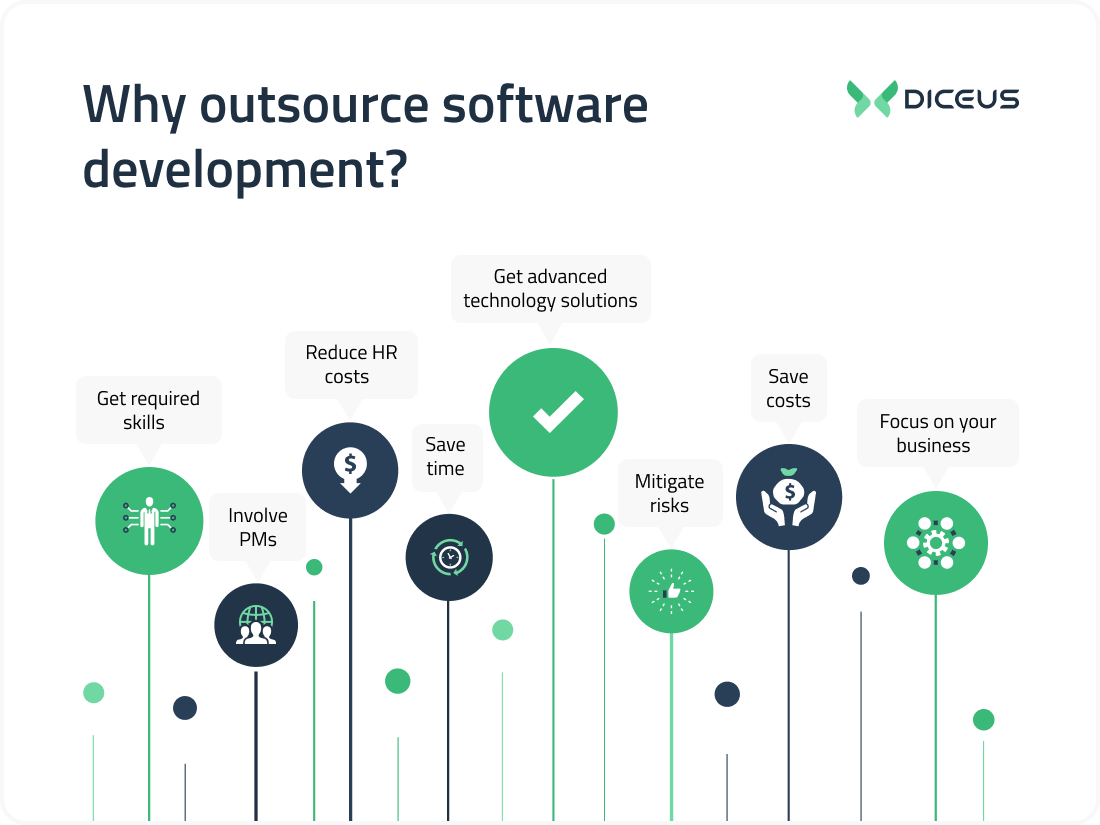
Focus on core processes and goals. While the third-party team is carrying out general development tasks, the in-house staff handles higher priority issues.
Release your product on the global marketplace faster than an in-house team would be able to develop software in the first place. Using outsourcing, you speed up the release of the project and monetize the product faster.
Reduce costs. As mentioned above, outsourcing helps save the budget by hiring workers at a lower rate, eliminating the costs of setting up the workplace, and recruiting employees.
Outsourcing risks are only potential and can be completely eliminated by selecting a team carefully. By the way, as we mentioned, 78% of companies have a positive attitude towards outsourcing cooperation.
What makes a country good for outsourcing
This article lists the countries based on the key criteria for choosing a contractor for outsourcing. Considering this information and project goals when making your choices, you can easily determine where to outsource software development most efficiently for your particular case.
Technical education level of the resources
Investment in corporate education is one of the main criteria by which companies choose a contractor to delegate tasks to outsourcing. According to statistics, good secondary and higher education is provided in the countries of Eastern Europe and Central Asia. Mathematics and computer science reach the highest development at universities in Latin America, particularly in Argentina.
Location and time zone differences
It’s important to consider time zone differences when collaborating based on an outsourcing model. However, contractors tend to work in a team with a manager who facilitates communication with the rest of the team. Maintaining communication is an effective practice for project development and successful collaboration at any stage of development.
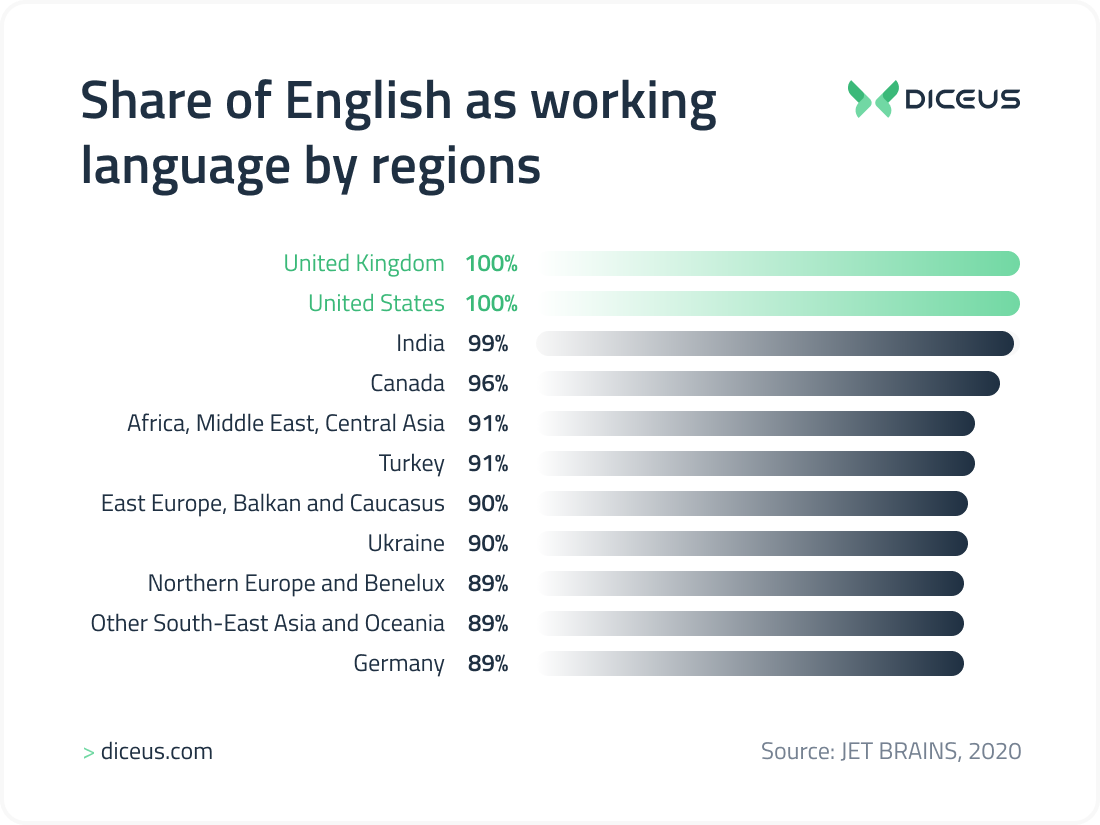
English language skills
Most outsourcing developers (in the countries mentioned in the article) speak English at the Intermediate and Upper-Intermediate levels. Advanced English is a must for positions such as Team Lead and Project Manager. In the list of the 10 best countries to outsource software development, we describe in which countries English is the second language after the native one and where you can find developers without fear of the language barrier.
Project complexity experience
India is among the leaders in the number of IT professionals with extensive knowledge of programming languages. The main tasks that are delegated to outsourcing contractors here are related to the development of mobile applications.
Companies based in the United States outsource 80% of projects to developers from Ukraine. Ukraine ranks first in C ++ and Unity3D, second in Magento, JS, and Scala, and third in Ruby, Python, Node.js, and ASP.NET in terms of the number of developers in the global market.
Outsourcing specialists from Poland mainly handle the tasks of creating and supporting the user interface, as well as internal product development.
Resources availability and tech competency diversity
Over 100,000 IT specialists work in Ukraine today, of which more than 60% offer their services based on an outsourcing model. It is the leading country in terms of the number of C++ and Unity3D software developers. You can also find extensive developer experience with JavaScript, Scala, Magento, PHP, Node.js, Ruby, ASP.NET, Python, and frontend technologies.
In Poland, from 250,000 developers, only 20% work for outsourcing with the dominant programming languages Java, Python, and Ruby.
India’s IT outsourcing market with 3 million developers focuses on C++, Java, and web development. Machine learning, artificial intelligence, and robotics are of equal interest in the country.
Cultural differences in IT outsourcing countries. Do they matter?
Local culture also influences how people work and communicate with each other. Consider possible cultural differences when selecting employees. Is it customary in their countries of origin to work on weekends or on public holidays?
In terms of the “Western” approach to business project management, Argentina, Poland, Ukraine, and most of the Eastern European countries will come closest to the expected approaches to work, the level of productivity, and manners of communication.
How to deal with cultural differences and communication issues
How to conveniently work with teams from outsourcing countries? And how to overcome cultural differences when working with an offshore/onshore outsourcing contractor?
- Settle all the conditions beforehand. Before getting to work, discuss all the details and set clear requirements, and share your expectations for cooperation.
- Approach new team members with respect. Try to adapt your communication and working methods to suit both parties. Treat everybody’s culture with the same respect you treat your own culture.
Transferring responsibilities to outsourcing doesn’t mean briefly talking about the project and believing that specialists will figure out the details on their own. Maintain feedback and promptly report any changes in the project – this guarantees successful, fruitful cooperation.
You may find this interesting: “How to manage IT outsourcing risks”
10 world top outsourcing countries
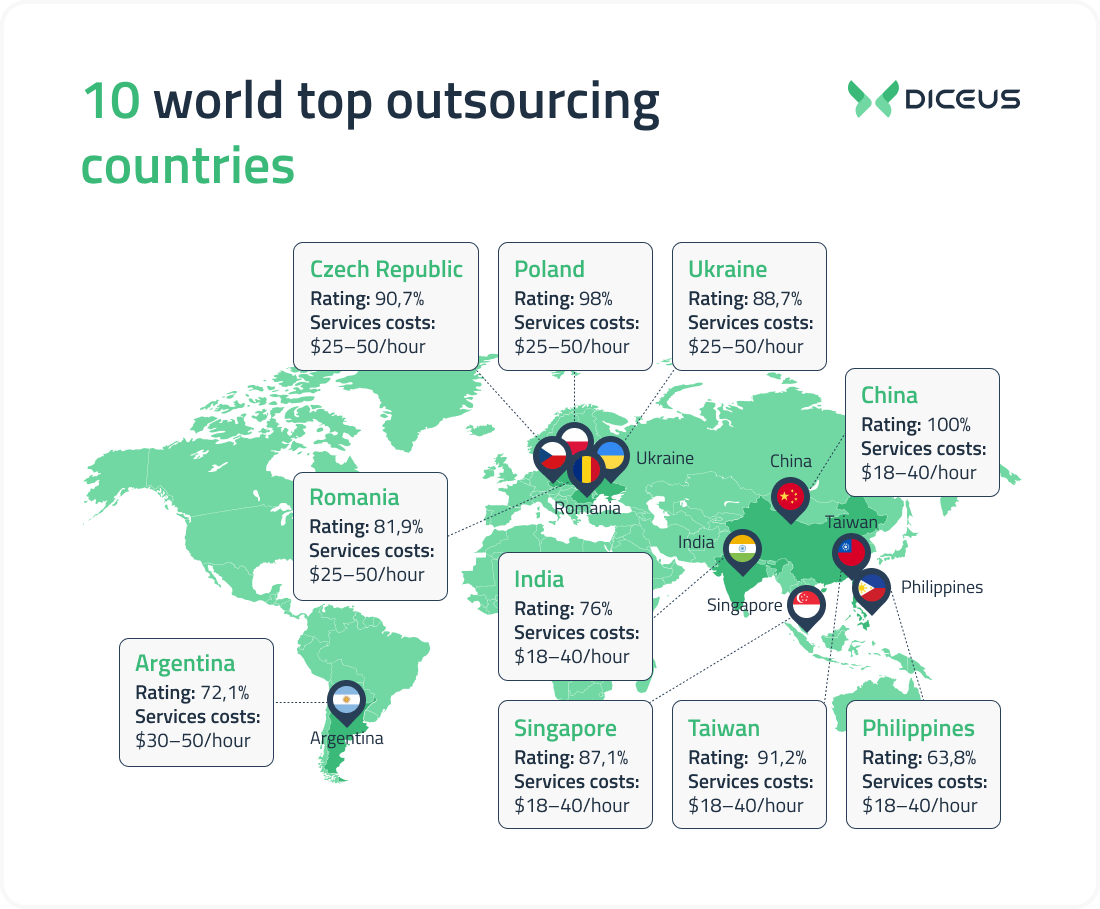
The data may differ, but in most lists of the best countries to outsource software development, you can most often find Ukraine, Poland, Argentina, India, China, and some other locations. We will compare the advantages and disadvantages of outsourcing to some of these countries based on factors such as:
- Tech skills
- Rating
- Services costs
- Command in English
- Time zone difference
Without further ado, here’s our take on the best countries for outsourcing.
Poland
Tech skills of local specialists: PHP is the most used programming language. Python, Shell, Ruby, Java, and the use of the .NET Framework are equally common. IT education in Poland is in demand and attracts students from all over Europe.
Rating (according to HackerRank): 98%.
Services costs (data from PayScale and Glassdoor): $25–50/hour.
Command in English (according to EF EPI): upper-intermediate and advanced, with a regional rating of 63,76. English is the second language for 30% of the country’s population.
Time zone: GMT+2.
According to HackerRank statistics, Poland is the third location in the world with the biggest number of in-depth experienced programmers.
Ukraine
Tech skills of local specialists: IT companies and outsourcing teams of Ukraine focus on Big Data and AI, mobile development, blockchain technologies, and cryptocurrency-related projects. The position of Senior developers is very popular. The most common programming languages are C++, Java, Ruby, and PHP. Ukrainians acquire their technical skills mainly via specialized courses. Some companies offer free training with the possibility of further employment as well.
Rating (according to HackerRank): 88,7%.
Services costs (data from PayScale и Glassdoor): $25–50/hour.
Command in English (according to EF EPI): basic, with a regional rating of 52,13.
Time zone: GMT+3.
Check out how to hire developers in Ukraine now.
Czech Republic
Tech skills of local specialists: Corporate taxes in the country are only 19%, and EU investments and grants are further supporting the growth of the IT sector. Brno alone, the second-largest city after Prague, graduates more than 15,000 STEM students annually. The top 3 programming languages here are Java, JavaScript, and PHP.
Rating (according to HackerRank): 90,7%.
Services costs (data from PayScale and Glassdoor): $25–50/hour.
Command in English (according to EF EPI): upper-intermediate, with a regional rating 59,30.
Time zone: GMT+2.
Romania
Tech skills of local specialists: The IT outsourcing market in Romania is much smaller than the market of Ukraine and Poland. However, the country has 116,000 IT professionals, most of whom work from Cluj-Napoca, Bucharest, and Timisoara. The level of English proficiency among specialists is 61.36 points, which is a good indicator. In addition, Romania is located at the intersection of the time zones of Western Europe and the United States, which makes it easy to interact with software developers.
Rating (according to HackerRank): 81,9%.
Services costs (data from PayScale and Glassdoor): $25–50/hour.
Command in English (according to EF EPI): upper-intermediate and advanced, with a regional rating 61,36.
Time zone: GMT+3.
India
Tech skills of local specialists: India is one of the leading IT outsourcing countries. The most popular areas in development are applications and products related to Microsoft technology, as well as open-source projects that are powered by machine learning, AI, and blockchain.
Despite the relatively low level of literacy, the government pays attention to the conduct of mathematical research and the development of technologies. The country is introducing a Western approach to education, and up to 2.6 million STEM (Science, Technology, Engineering, Math) specialists graduate from universities every year.
Rating (according to HackerRank): 76%.
Services costs (data from PayScale and Glassdoor): $18–40/hour.
Command in English (according to EF EPI): intermediate, with a regional rating 55,49.
Time zone: GMT+5:30.
China
Tech skills of local specialists: The country launches top software products based on Python, Shell, as well as projects related to Big Data. The focus in primary and secondary education is primarily on mathematics and high tech.
Rating (according to HackerRank): 100% (top of the best IT outsourcing countries).
Services costs (data from PayScale and Glassdoor): $18-40/hour.
Command in English (according to EF EPI): intermediate, with a regional rating of 53.44. However, only 10 million of China’s 1.3 billion people speak English.
Time zone: GMT+8.
The outsourcing services market in China is growing by 30% annually. Among the disadvantages of cooperating with specialists in the country — China often doesn’t comply with intellectual property laws. To mitigate the risks, it is recommended to hire a well-rated outsourcing team and establish clear rules before starting the project.
Argentina
Tech skills of local specialists: Argentina-based outsourcing companies have a huge background in software and mobile development, data migration to the cloud, and DevOps engineering expertise. Free education in the field of computer technologies is available in the country, as well as individual programs in the school plan. Already during their studies at universities, 70% of students combine study with work.
Rating (according to HackerRank): 72,1%.
Services costs (data from PayScale и Glassdoor): $30–50/hour.
Command in English (according to EF EPI): upper-intermediate, with a regional rating 58,38.
Time zone: GMT-3.
Philippines
Tech skills of local specialists: English is the official language in the Philippines, and software developers speak it fluently. Contractors from the Philippines are noted to work faster than experts in other Asian countries. More than 190,000 technical experts work in the country, according to PISA (Philippine Software Industry Association). This number is expected to reach 210,000 by 2022.
Rating (according to HackerRank): 63,8%.
Services costs (data from PayScale and Glassdoor): $18–40/hour.
Command in English (according to EF EPI): advanced, with a regional rating of 60,14.
Time zone: GMT+8.
Taiwan
Tech skills of local specialists: The Taiwan government has set a goal to turn the country into an Asian Silicon Valley. In 2020, the country is actively introducing STEM education, and interest in innovation and technology has allowed the island to become one of the leading technology countries in the world. The local professionals are known for their hard-working spirit, firm work ethics, and higher quality of work than the representatives of most other Asian countries. Despite the democratic prices for services, be prepared to face communication problems – Taiwan is ranked 40th among 80 countries in terms of English proficiency.
Rating (according to HackerRank): 91,2%.
Services costs (data from PayScale and Glassdoor): $18–40/hour.
Command in English (according to EF EPI): intermediate, with a regional rating of 54,18.
Time zone: GMT+8.
Singapore
Tech skills of local specialists: Most large outsourcing deals in Singapore involve the public sector, manufacturing, or financial services. IT services are dominated by work with cloud technologies, migration to the cloud, etc. According to a 2019 survey by W.Media, the key IT projects were Datacenter Migration and Expansion (40%) and Migration to Public or Hybrid cloud (11%).
Rating (according to HackerRank): 87,1%.
Services costs (data from PayScale and Glassdoor): $18–40/hour.
Command in English (according to EF EPI): upper-intermediate and advanced, with a regional rating 66,82.
Time zone: GMT+8.
How much does outsourcing software development projects to these countries cost?
While in the United States developers’ hourly rates vary from $70 to $180, most rates for software development abroad are much lower. They are determined by the experience, skills of the employee, and the average level of wages in the country. These are typical costs of services in the best offshore software development countries according to PayScale and Glassdoor. Please note that these are average figures.
| Role | Ukraine, Poland, Czech Republic, Romania | Argentina, Mexico, Brazil, Peru | India, Philippines, Taiwan, Singapore, China |
|---|---|---|---|
| Senior Software Engineer | $30 – $60 | $32 – $65 | $24 – $48 |
| Junior Developer | $22 – $31 | $28 – $57 | $20 – $30 |
| Senior QA (manual) | $19 – $57 | $45 – $60 | $20 – $37 |
| Junior QA (manual) | $18 – $30 | $30 – $40 | $15 – $23 |
| Business Analyst | $30 – $67 | $23 – $40 | $20 – $45 |
| Graphic Designer | $25 – $57 | $45 – $65 | $20 – $50 |
| DevOps | $40 – $61 | $38 – $60 | $20 – $39 |
| Project Manager | $25 – $60 | $41 – $68 | $24 – $50 |
What is the best country to outsource software development?
Countries of Eastern Europe are always included among the best countries for outsourcing — with a high level of knowledge in software development and affordable costs for services. Eastern European locations are the closest in mentality to the Western market, which guarantees more productive cooperation, even despite the time difference.
DICEUS is among the leading outsourcing companies with offices in Poland, Austria, Ukraine, Denmark, Lithuania, USA, UAE, and KSA. We have more than 100 completed projects under our belt, and over 250 certified specialists in the field of business analysis, development, design, and QA engineering in staff.
If you are looking for a reliable partner who is ready to share the company’s mission and develop your business by implementing the best solutions, please share your project ideas with our experts.
Best countries for outsourcing: Takeaways
Central and Eastern European (CEE) IT professionals are highly regarded in the outsourcing market. An extensive background in the fields of application development and work on machine learning, IoT, and AI, combined with a high quality of work and a Western form of thinking, make these countries very attractive to clients from the US, the UK, and other countries.
How to find outsourced developers
When choosing a country for outsourcing software development, pay attention to costs, adaptability of the IT market, quality of educational infrastructure, cultural differences, and so on.
When choosing an outsourced software development team, pay attention to whether the contractor understands your company’s mission and goals. You choose people not just for economy or convenience – you choose people who will help you achieve your project goals and grow your brand.
We’ll be glad to help you with your outsourcing needs. Book a free call!
FAQ
Despite recurring lockdowns due to the global COVID-19 pandemic, IT outsourcing keeps gaining popularity among Western companies. The market is expected to reach $98 billion by 2024. Moreover, the working-from-home paradigm seems to stimulate both outsourcers and their clients for further development: global spending on IT outsourcing has increased by 13.6% in comparison with the same period of 2020.
The global IT outsourcing market is not homogeneous. While the clients belong to the developed Western countries where IT staff is relatively expensive the most popular outsourcers are distributed among CEE, Argentina, India, and some countries of South-East Asia. The following FAQs can help determine the key criteria of selecting the top outsourcing countries for software development.
What are the best countries for outsourcing?
Even though contemporary labor resources are becoming more and more liquid (aka spatially irrelevant) due to digital communication channels there are certain places where IT staff of various specialties are geographically clustered. Some historical conditions of why one or another region belongs to the top outsourcing countries are available.
They are not numerous but quite specific. Population, for instance, is not the characteristic worth taking into consideration: it is barely reasonable to look for well-trained IT specs in both underpopulated Mongolia and overpopulated Nigeria. Instead, the national education level (especially the number of STEM graduates), available industrial infrastructure, English language skills of staff, and cultural proximity to the Western mentality all determine the place of a particular location among the top countries to outsource software development.
Which are the main criteria for choosing a country for outsourcing?
The following factors are widely considered the main selection criteria when you need to share your project with an IT outsourcing partner from abroad. Some of them differ not too much from region to region while others do significantly. Figuring out which of the factors is the most decisive one depends on peculiar features of your project.
The technical education level of the resources
This factor impacts the entire image of every outsourcing country in a way that little else does: no highly skilled professionals, no outsourcing business. There are many ways to assess the technical education level of staff in one or another country. National statistics on the number of university graduates can reveal how many STEM students swell the ranks of junior developers every year.
Another sort of national stats can show how many technology-oriented universities are available in the country. Besides, there are many international data providers that aggregate information about software development companies available in those countries that claim to be the leading IT outsources in the world. Hence, the more highly qualified IT specs are available in a certain location the bigger the chance the location belongs to the top outsourcing countries.
Location and time zone differences
This factor might seem to be irrelevant in the present days of digital communication. However, many indirect indicators of the feasibility of outsourced projects are hidden in the location info. The time zone difference is the first thing to be considered inter alia. True trouble-free communication between outsourcers and their customers takes place when working hours overlap albeit partially in both locations.
Even climate conditions can influence cooperation between outsourcers and customers to some extent. Both severe winters and super-hot summers require appropriate working facilities the cost of which can impact the cost of outsourcing services as a whole. That’s why studying the location peculiarities of your potential outsourcing partner is never redundant to make feasible decisions regarding top outsourcing countries.
English language skills
Since English is accepted as the language of international communication the qualification in English is crucial for those outsourcers who offer their services abroad. This is especially relevant in the context of software development: libraries, repositories, tutorials, and manuals, that is to say, the entire software terminology belongs to English syntax.
Insufficient skills in the English language can destroy mutual understanding between customers and outsourcing service providers. This is one of the biggest risk factors that should be thoroughly mitigated before any project starts. Team leads, project managers as well as all senior-level developers must possess advanced English to be competitive in the global outsourcing market in 2022. Service providers from top outsourcing countries meet this requirement usually.
Project complexity experience
However big words outsourcers can use to promote their services only confirm practical experience in complex projects is the true indicator of professional skills. A portfolio of successful use cases validated with real customers is what should be considered when one or another outsourcing provider is assessed.
There are a couple of factors to be taken into consideration in this context: the clientele geography in terms of locations of the customers and the technologies with which outsourcers work. It’s so happened historically that the top outsourcing countries differ in technologies their outsourcing providers offer. Singapore is good at data center migration, for example, while Ukraine is great at C++, Java, Ruby, and PHP. Besides, if a service provider’s portfolio shows main outsourced countries like the USA and Western Europe the trustworthiness of such an outsourcer should hardly be questionable.
Resources availability and tech competency diversity
This factor overlaps with the technical education level discussed above. The available labor resources should correspond to the status of a top outsourcing country. It means a sufficient number of well-qualified IT experts is a must for the country claiming to be a leading outsourcer of software development services.
The diversity of tech competencies increases the chance of any outsourcer to raise interest among potential customers. Both available universities and the national technical infrastructure determine what technologies are well developed in one or another outsourcing location. The high-tech industries such as aircraft building, rocket science, ship construction, and the like indirectly hint at the competencies inherent in local labor resources. Ukraine is a prominent example of tech diversity in this regard.
Cultural differences in IT outsourcing countries
Despite being unobvious, culture has a lot to do with business in general and IT outsourcing in particular. People are different in mentality. As Samuel Huntington said wars have erupted not between countries but between cultures. The thesis is worth taking into account when selecting your future outsourcing partner.
Of course, such a standalone activity as software development is unifying interactions between participants with common tech syntax to some extent. However, people who historically share similar categories at the social and cultural levels can come to mutual understanding much easier than the ones whose mental archetypes stand far away from each other. Since the majority of outsourcing customers reside in the Western world the top outsourcing countries from CEE appear in a more favorable position in the context of mental proximity than outsourcers from other regions do.
Which country has more IT companies?
The Global Services Location Index (GSLI) ranks India as the leading outsourcing provider in the world in terms of both the availability of labor resources and financial attractiveness. This means that there are a lot of Indian companies that offer various outsourcing services for a considerable price.
However, quantitative analysis alone can unlikely show the full picture of the IT outsourcing market. The selective criteria given above can help apply qualitative considerations to the subject. If 80% of about 100K well-qualified IT specs working in Ukraine provide services to customers from the United States, then something more than resource availability and hourly tariffs should be taken into consideration to figure out top outsourcing countries. It is definitely better to have a narrower choice between the best IT outsourcing providers than a wider one between just average outsourcers.





